

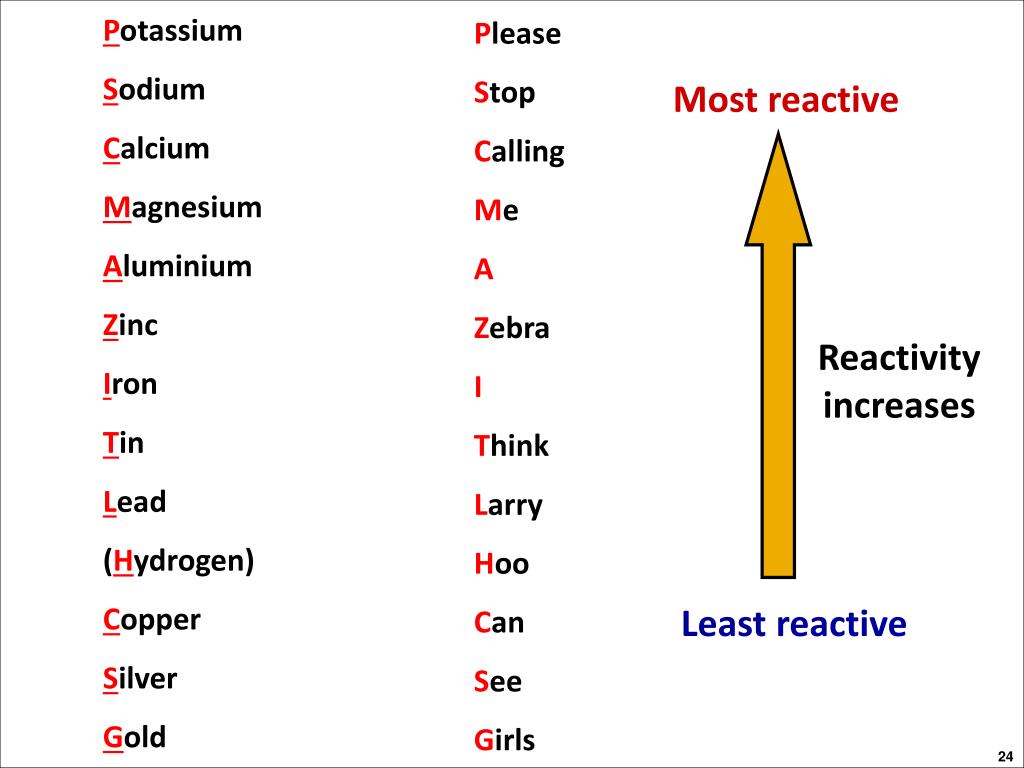
However, copper can be extracted using carbon or hydrogen. Note that zinc and iron can be displaced from their oxides using carbon but not using hydrogen. Here is the reactivity series including carbon and hydrogen: It is useful to place carbon and hydrogen into the reactivity series because these elements can be used to extract metals. When this layer is removed, the observations are more reliable. Carbon and the reactivity series mnemonic. The metals at the top of the reactivity series are the most reactive and the metals at the bottom are the least reactive. This is because its protective aluminium oxide layer makes it appear to be less reactive than it really is. Note that aluminium can be difficult to place in the correct position in the reactivity series during these experiments. The quicker the fizzing, the more reactive the metal. The speed at which hydrogen bubbles are produced tells us how reactive a metal is with acid. The reactivity of metals is due to their incomplete electronic. It is also known by the name activity series. Therefore, the term reactivity series refers to a series of metals arranged in descending order of reactivity. The tables show how the elements react with water and dilute acids: Element Reactivity series is an arrangement of metals from highest to lowest reactivity order. Observations of the way that these elements react with water, acids and steam enable us to put them into this series. For example: P eople S ay L ittle C hildren M ake A Z ebra I ll C onstantly S niffing G iraffes. The reactivity series of metalsĪ good way to remember the order of a reactivity series of metals is to use the first letter of each one to make up a silly sentence. The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities. More reactive metals have a greater tendency to lose electrons and form positive ions. Please visit and I hope it will help in your teaching.In a reactivity series, the most reactive element is placed at the top and the least reactive element at the bottom.
#Reactivity series mnemonic free#
This is a completely free site and requires no registration. This powerpoint was kindly donated to is home to over a thousand powerpoints submitted by teachers. The Reactivity Series Increasing reactivity Potassium Sodium Calcium Magnesium Aluminium Carbon Zinc Iron Lead Copper Silver Gold The Reactivity Series lists metals in order of reactivity: Nickel + hydrochloric acid Lithium hydroxide + hydrogen Lithium chloride + hydrogen Silver oxide Magnesium sulphate + hydrogen Potassium oxide Aluminium oxide Manganese oxide + hydrogen Sodium sulphate + hydrogen Lithium oxide Nickel chloride + hydrogen.Lithium + sulphuric acid When a metal reacts with an acid it gives off hydrogen (which can be “popped” using a lit splint).magnesium + hydrochloric acid magnesium chloride + hydrogen Reactions of metals with acids METAL + ACID SALT + HYDROGEN e.g.

Concept: Activity series of metals Mnemonics: Popular Scientists Can Make A. The other product will be either a metal hydroxide or a metal oxide. Fe + CuSO4 Reactivity or activity series FeSO of metals 4 + Cu : All. Iron + steam When a metal reacts with water hydrogen is always given off.Reactions of metals with water METAL + WATER METAL OXIDE + HYDROGEN METAL + WATER METAL HYDROXIDE + HYDROGEN We can make this reaction happen quicker by burning the metal.


 0 kommentar(er)
0 kommentar(er)
